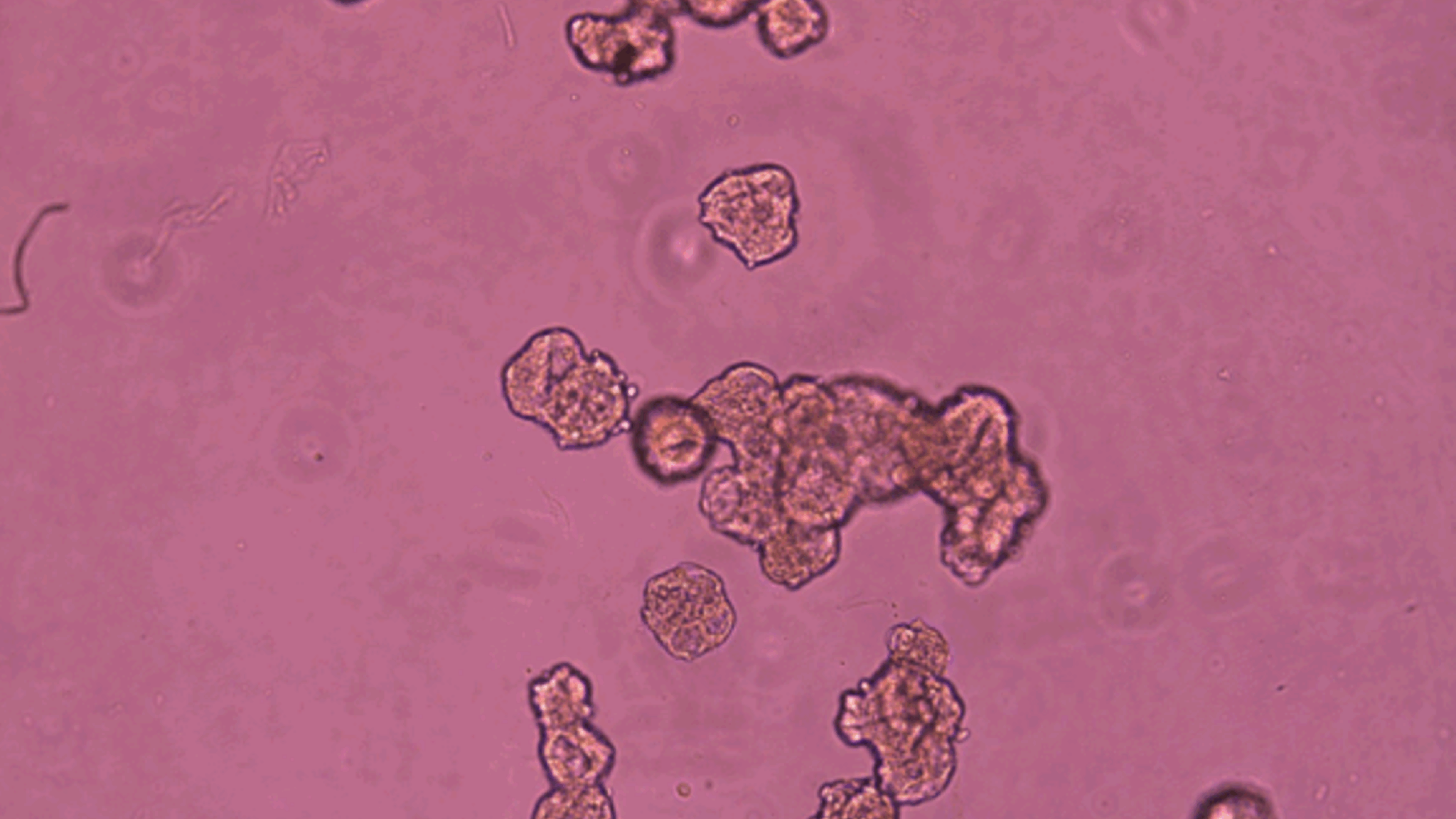
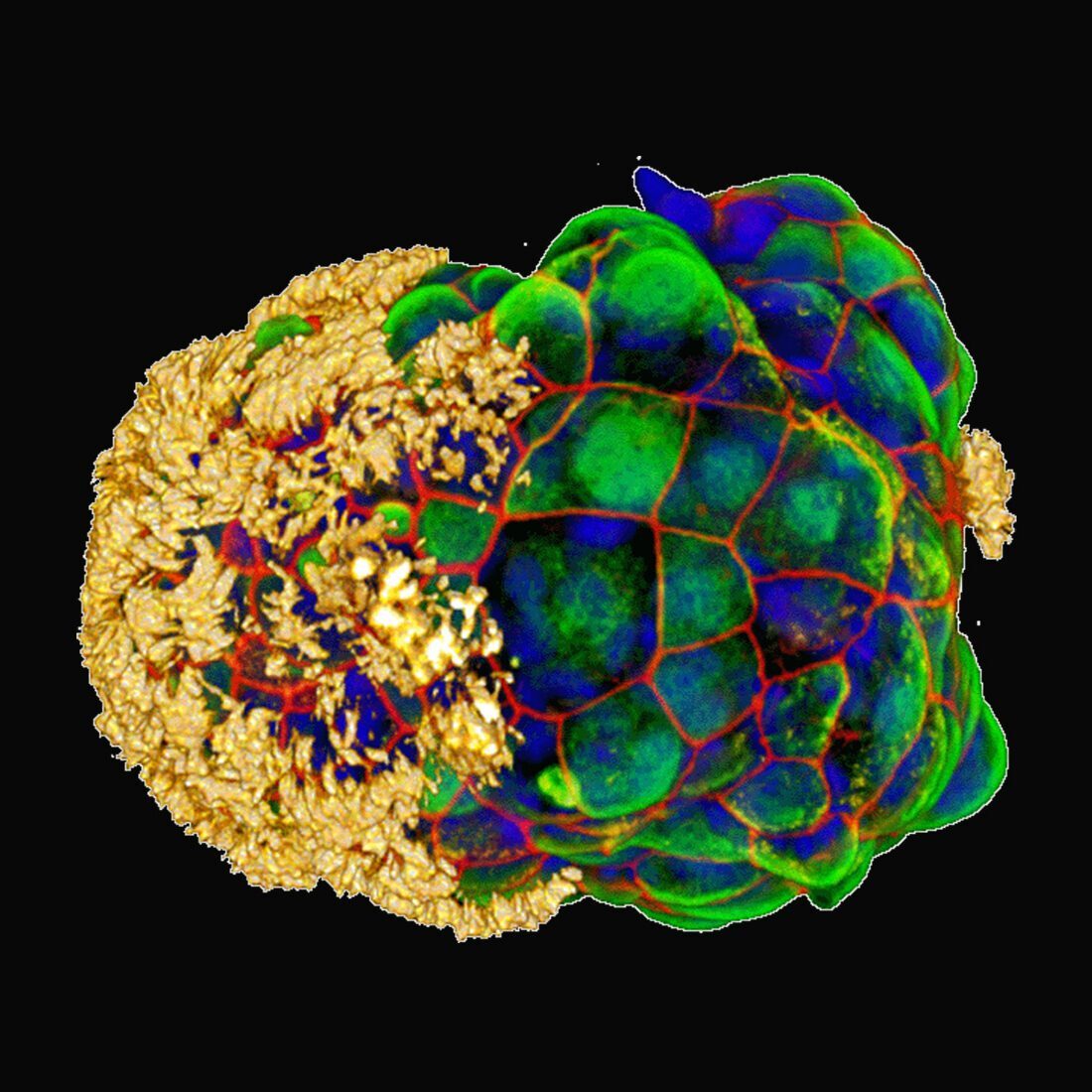
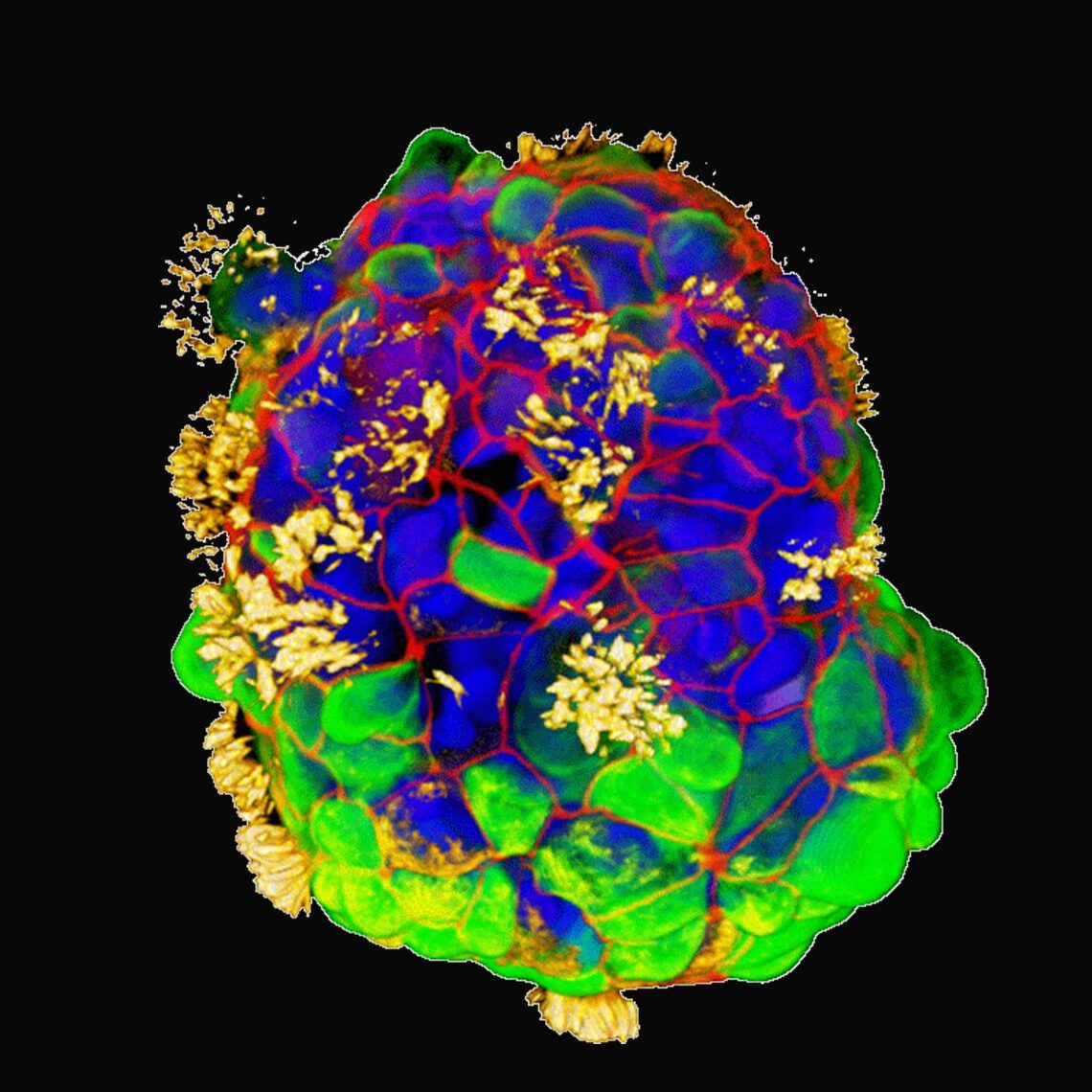
It reads like a classic tale of a mid-life crisis: a radical change of scenery frees our protagonist from regular duties and structures, allowing them to find new ways of being and interacting, becoming, in a real sense, younger than they once were. In this case, though, the protagonists are human cells, taken from a donor’s trachea and given a new life in a non-stick Petri dish, where they can cluster into freewheeling, cilia-covered cellular spheroids called Anthrobots.
![]()
Since their debut in 2023 as Gizem Gumuskaya’s doctoral project in Michael Levin’s lab at Tufts University, Anthrobots have have been helping biologists broaden their understanding of how cells function in their environment, and the ways that groups of them can undergo significant changes in form and function even though their underlying genomes remain unaltered.
Anthrobots are created by placing the donor cells in a semisolid gel where they can divide and cluster for a couple of weeks. Then the gel is dissolved and over the course of a few more days the cells reorient themselves, switching their cilia to the outside to create little swimming spheres in a couple of standard patterns. Thus freed, they behave quite differently than their forebears, moving through their new medium at various speeds and, remarkably, facilitating the healing of damaged nerve cells they encounter.
In a new study published in Advanced Science, Gumuskaya and Levin explore what their Anthrobots have been up to since their debut, looking specifically at how they change their genetic expression in light of their new lifestyle. They discovered that the Anthrobots had changed the expression of more than 9,000 genes, representing almost half of their genome. This included activating some of their most ancient genes (ones shared with humans’ single-celled ancestors) as well as embryonic genes that guide emerging symmetry and folding of cells and tissues.
This reverse-genetic-time-travel wasn’t just in terms of function.
Our cells run on a biological clock that can progress at a different rate than our actual age.
Other studies have established ways of calculating an organism’s epigenetic age, based on the changing patterns of DNA methylation at specific locations of the genome.
The 21-year-old donor who provided the seed cells for the Anthrobots had an epigenetic age of 25. But as the Anthrobot cells adjusted to their new environment and structure, they rolled back their epigenetic clock to 18.7 years — making them biologically 25% younger than the cells of origin.
Levin thinks that as their Anthrobot lifestyle activates developmental genes and guides the cells into novel forms, the cells may be receiving signals consistent with embryogenesis, which conflicts with their actual age. “So they end up reversing some of the DNA markers of aging to be consistent with their current state,” Levin says.
It’s a fascinating finding, and as with other aspects of Anthrobot life, it has potential to help us understand more about developmental diseases such as birth defects, and learn how to help grow new tissues and even organs for regenerative medicine (the Anthrobots themselves can regenerate, self-repairing from minor damage like being stabbed with a hypodermic needle). And although they are made of human cells, studies with Anthrobots can be ethically advantageous research subjects: they are brainless, patient-specific, not derived from embryos, and free from genetic modifications or animal material.
Perhaps the adage that “you’re only as old as you feel” has a germ of truth to it, even at the cellular level.
Still Curious?
Read “The Morphological, Behavioral, and Transcriptomic Life Cycle of Anthrobots” and the original Anthrobots paper, “Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells”
Nate Barksdale writes about the intersection of science, history, philosophy, faith, and popular culture. He was editor of the magazine re:generation quarterly and is a frequent contributor to History.com.